Don't miss our holiday offer - up to 50% OFF!
The Complete Guide to Research Chemical Classification & Nomenclature: Mastering NPS Structures, Naming & Trends in 2026
In the fast-evolving world of research chemicals and novel psychoactive substances (NPS), understanding research chemical classification and research chemical nomenclature is essential for researchers, forensic toxicologists, pharmacologists, and regulatory professionals. As new compounds emerge monthly, precise categorization and standardized naming systems enable accurate identification, legal tracking, and scientific communication. This complete guide to research chemical classification & nomenclature explores the frameworks used in 2026, key classes, naming conventions, practical applications, and emerging trends shaping the field.
Whether you’re studying tryptamine research chemicals, phenethylamine compounds, arylcyclohexylamines, or dissociative research chemicals, mastering research chemical classification and NPS nomenclature helps navigate the complex landscape of synthetic analogs. With over 1,200 NPS documented by the UNODC Early Warning Advisory since 2009, and new variants appearing in dark web markets and seized samples, this guide provides the clarity needed for reliable analysis and compliance.
What Are Research Chemicals? Defining the Scope
Research chemicals are synthetic compounds produced for laboratory investigation, often analogs of controlled substances or pharmaceuticals. Unlike approved drugs, they lack extensive clinical data and are sold “not for human consumption” to evade regulation. The term novel psychoactive substances (NPS) is more specific, referring to psychoactive research chemicals that mimic traditional drugs (cannabinoids, opioids, stimulants, hallucinogens) while avoiding legal controls.
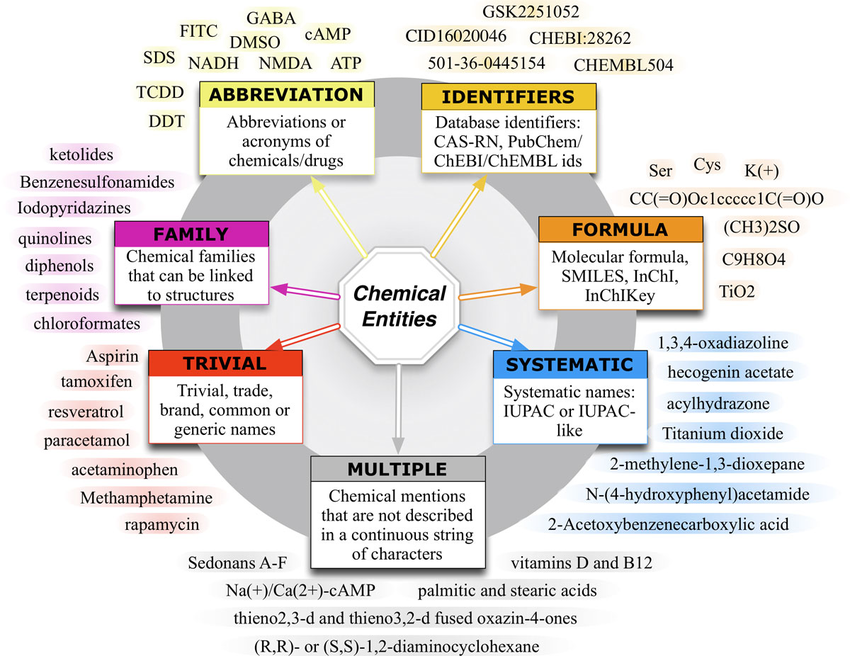
Research chemical classification organizes these compounds by chemical structure, pharmacological effect, or legal status. Research chemical nomenclature standardizes names using IUPAC rules, common abbreviations, and street slang. Accurate novel psychoactive substances nomenclature is critical for forensic reporting, database entries, and peer-reviewed publications.
In 2026, research chemical classification systems have become more sophisticated, incorporating AI-assisted structural prediction and metabolomics data to anticipate new analogs before they appear in markets.
Core Systems for Research Chemical Classification
Research chemical classification typically follows three main approaches:
- Structural Classification Groups compounds by molecular scaffold:
- Tryptamines (indole ring + ethylamine)Phenethylamines (benzene ring + ethylamine)Arylcyclohexylamines (PCP-like, cyclohexane + amine)Piperazines & cathinones (beta-keto amphetamines)
- Pharmacological Classification Based on primary receptor targets:
- Serotonergic hallucinogens (5-HT2A agonists)
- Dopaminergic stimulants (DAT inhibitors)
- Opioid receptor agonists
- GABAergic sedatives
- Legal/Regulatory Classification UNODC and EMCDDA categorize by risk:
- Class A/B/C (UK)
- Schedule I/II/III (US)
- Temporary class drugs (EU)
- Research chemical classification by legal status drives enforcement and lab protocols.
In 2026, hybrid systems combine structural and pharmacological data for predictive modeling, helping anticipate new emerging NPS classes.
Research Chemical Nomenclature: Rules, Conventions & Best Practices
Research chemical nomenclature follows IUPAC guidelines for systematic naming, but the field uses multiple layers:
- IUPAC Systematic Name: Precise, e.g., 2-(4-fluorophenyl)-2-(piperidin-2-yl)acetate for 4F-MPH.
- Common/Street Names: 4F-MPH, 4F-methylphenidate.
- Abbreviations: 4F-MPH, 3-FMC.
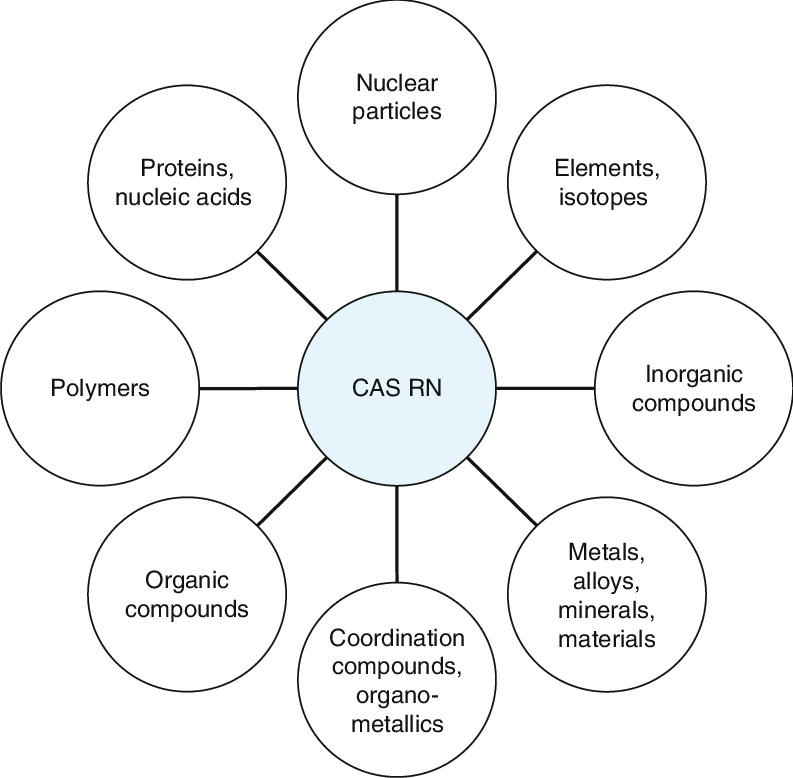
- CAS Registry Number: Unique identifier (e.g., 1354631-33-6 for 4F-MPH).
- INCI/Pharmacopeia Names: Rarely used for research chemicals.
NPS nomenclature often includes positional isomers (2C-B vs 2C-I), ring substitutions (3-MMC vs 4-MMC), and functional groups (2-oxo-PCE for O-PCE). Confusion arises when labs use vendor names or slang—standardizing research chemical nomenclature with IUPAC + CAS is critical for forensic reports.
In 2026, databases like PubChem and NPS Discovery mandate IUPAC + CAS for NPS nomenclature, reducing errors in case reporting.
Major Classes of Research Chemicals: Structural & Pharmacological Breakdown
Research chemical classification by scaffold reveals patterns:
- Tryptamines Indole-based, 5-HT2A agonists. Examples: 5-MeO-DMT, 4-HO-MiPT. Tryptamine research chemicals dominate psychedelic studies.
- Phenethylamines Amphetamine-like. 2C-X series (2C-B, 2C-E), DOx (DOB, DOI). Phenethylamine compounds are key for entactogen research.
- Arylcyclohexylamines Dissociatives. PCP, ketamine, 3-MeO-PCP. Arylcyclohexylamine research chemicals focus on NMDA antagonism.
- Synthetic Cathinones Stimulants. 3-MMC, α-PVP. Cathinone NPS drive forensic demand.
- Synthetic Cannabinoids CB1 agonists. ADB-BUTINACA, MDMB-4en-PINACA. High toxicity risk.
- Opioid NPS Nitazenes, fentanyl analogs. Extreme potency.
- Benzodiazepine Analogs Flualprazolam, etizolam. Sedative NPS.
- Piperazines & Novel Stimulants BZP, TFMPP. Less common now.
Each class has unique research chemical nomenclature challenges—e.g., positional isomers in phenethylamines require isomer-specific reporting.
Practical Applications of Research Chemical Classification & Nomenclature
Research chemical classification and research chemical nomenclature enable:
- Accurate forensic identification in seized samples
- Development of detection methods (LC-MS/MS, GC-MS)
- Toxicological profiling for overdose cases
- Pharmacological SAR studies
- Regulatory monitoring via early warning systems
In 2026, AI tools predict new analogs based on research chemical classification patterns, aiding preemptive scheduling.
Challenges in Research Chemical Nomenclature & Classification
- Isomer confusion (positional, stereoisomers)
- Rapid emergence of new variants
- Vendor-specific naming
- Polydrug mixtures in samples
Standardization efforts (UNODC, EMCDDA) push for IUPAC + CAS in reporting.
Future Trends in Research Chemical Classification & Nomenclature 2026
- AI-driven nomenclature prediction
- Metabolomics for unknown NPS
- Global harmonization of NPS nomenclature
- Focus on hybrid compounds (e.g., cannabinoid-opioid)
Research chemical classification will increasingly integrate multi-omics data.
Conclusion: Mastering Research Chemical Classification & Nomenclature
This complete guide to research chemical classification & nomenclature equips you to navigate NPS research confidently. Accurate research chemical nomenclature and systematic research chemical classification are foundational for advancing forensic, toxicological, and pharmacological discovery in 2026.
Stay informed, prioritize safety, and contribute responsibly to this dynamic field.
FAQ: Research Chemical Classification & Nomenclature
1. Structural vs pharmacological classification? Structural groups by molecular backbone (tryptamines, phenethylamines, cathinones); pharmacological groups by primary effects/receptors (5-HT2A agonists, DAT inhibitors).
2. Why use IUPAC names? They provide one unambiguous, systematic name for every compound, eliminating confusion from slang, vendor names or isomers.
3. Most common naming mistake? Positional isomers (e.g., 2C-B vs 25I-NBOMe) and inconsistent abbreviations (3-FMC vs 3F-MC) cause frequent misidentification in reports.
4. How to cite a research chemical correctly? First mention: full IUPAC name + CAS (if known) + common abbreviation in parentheses. Example: 3-Fluoromethcathinone (3-FMC, CAS 123456-78-9).
5. Hardest classes to classify? Arylcyclohexylamines, NBOMe series, highly substituted cathinones – due to stereocenters, multiple naming conventions and rapid analog creation.
6. Are all research chemicals classified as NPS? No. NPS refers specifically to psychoactive substances not yet scheduled internationally. Many research chemicals (precursors, standards, non-psychoactive analogs) are not NPS.
7. How has AI changed classification & nomenclature? AI predicts analog structures, generates IUPAC names, matches spectra and flags isomers, helping forensic labs reduce misidentifications.
8. Where to find the latest NPS nomenclature resources? UNODC Early Warning Advisory, EMCDDA NPS database, PubChem, ChemSpider, NPS Discovery (CFSRE), Drug Testing and Analysis journal.
9. Is there one global classification standard? No single standard. Most labs use a hybrid of IUPAC structural naming + pharmacological grouping + CAS numbers.
10. Why is accurate nomenclature critical in court? Misidentification of isomers/analogs can lead to incorrect charges or case dismissals. Courts increasingly require IUPAC names, CAS numbers and validated analytical methods.
