Don't miss our holiday offer - up to 50% OFF!
🧪 GC-MS vs LC-MS: A Comparative Guide for Analytical Chemistry Applications
Introduction In analytical chemistry
Analytical research chemistry has evolved into one of the most sophisticated branches of modern science, providing researchers with the tools to separate, identify, and quantify chemical substances with remarkable precision. Among the most widely used techniques are Gas Chromatography–Mass Spectrometry and Liquid Chromatography–Mass Spectrometry — two methods that serve as the backbone of chemical and forensic analysis.
Both combine separation and detection technologies, but their differences in sample type, volatility, detection limits, and ionization methods make each more suitable for specific research purposes. Understanding these differences is crucial for scientists, analytical chemists, and laboratories selecting the right approach for their studies.
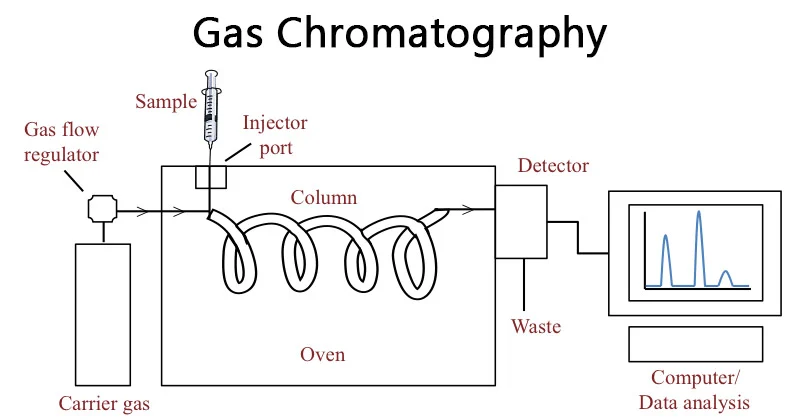
1. The Basics of Chromatography and Mass Spectrometry
At its core, chromatography is a separation technique that divides a mixture into individual components based on physical and chemical properties. Mass spectrometry (MS), on the other hand, identifies these components by measuring their mass-to-charge ratio (m/z).
When combined, the two techniques deliver a complete analytical workflow:
- Chromatography separates the mixture into distinct compounds.
- Mass spectrometry detects and identifies each compound based on its molecular mass and fragmentation pattern.
Both methods differ primarily in the type of chromatography they use:
- GC utilizes chromatography with a gaseous mobile phase.
- LC uses chromatography with a liquid mobile phase.
These differences determine the type of compounds that can be analyzed, the sample preparation requirements, and the resulting data quality.
2. What is GC-MS (Mass Spectrometry)?
This is one of the most established and precise analytical methods for identifying volatile and thermally stable compounds.
Principle
The sample is vaporized and carried by an inert gas (commonly helium) through a capillary column coated with a stationary phase. Each compound interacts differently with the stationary phase, leading to separation. As compounds exit the column, they enter the mass spectrometer, where they are ionized, fragmented, and detected according to their mass-to-charge ratio.
Key Components
- Injector: Introduces the vaporized sample.
- Capillary Column: Responsible for compound separation.
- Mass Spectrometer: Detects and identifies each analyte based on ion patterns.
- Detector: Produces chromatographic peaks for analysis.
Applications
It is widely used in:
- Forensic toxicology (identification of controlled substances and metabolites)
- Environmental testing (volatile organic compounds, pesticides)
- Food and flavor analysis
- Pharmaceutical purity assessments
Its high resolution and reproducibility makes this form of chromatography a gold standard for confirming compound identity in research chemistry.
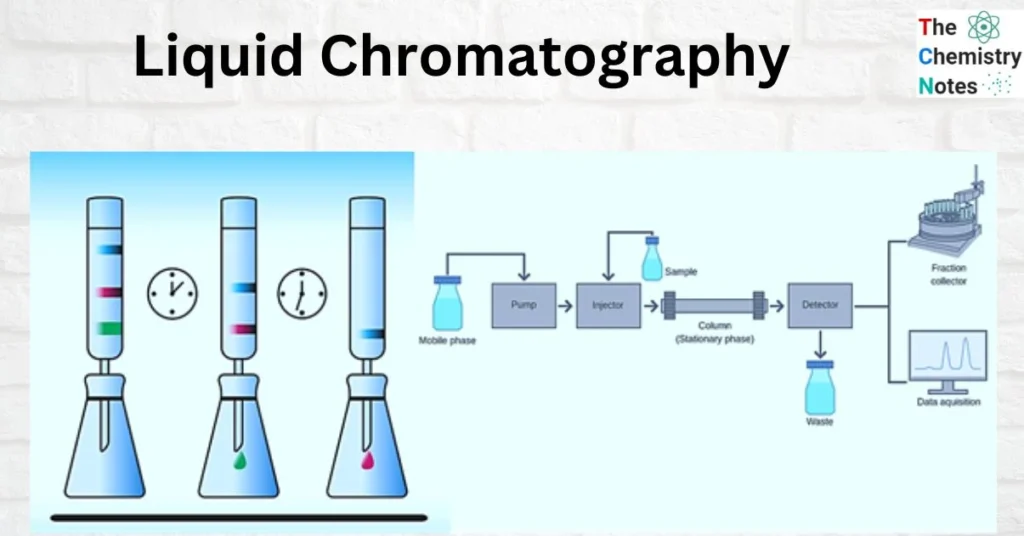
3. What is LC-MS (Mass Spectrometry)?
This is a more versatile technique capable of analyzing non-volatile, thermally labile, and polar compounds that are unsuitable for gas chromatography.
Principle
The sample, dissolved in a liquid solvent, passes through a column filled with a stationary phase. Compounds are separated based on polarity and chemical affinity. The eluate then enters the mass spectrometer, where molecules are ionized (commonly by Electrospray Ionization (ESI) or Atmospheric Pressure Chemical Ionization (APCI)) and detected according to their m/z ratio.
Key Components
- Liquid Pump and Injector: Control solvent flow and introduce samples.
- HPLC or UHPLC Column: Separates compounds by polarity or molecular size.
- Ionization Source: Converts molecules into ions for MS detection.
- Mass Analyzer: Identifies molecular masses and fragmentation patterns.
Applications
It has become indispensable in:
- Pharmaceutical research (metabolite profiling, drug discovery)
- Proteomics and metabolomics
- Forensic toxicology and doping control
- Environmental and biological sample analysis
This form of chromatography excels in analyzing polar or large biomolecules, providing excellent sensitivity and molecular-level insights.
4. Comparative Analysis
The table below summarizes the key differences between both techniques:
| Parameter | GC-MS | LC-MS |
|---|---|---|
| Mobile Phase | Gas (inert, e.g., helium) | Liquid (solvent mixture) |
| Analyte Type | Volatile, thermally stable | Non-volatile, thermally labile |
| Ionization Technique | Electron Ionization (EI), Chemical Ionization (CI) | Electrospray Ionization (ESI), APCI |
| Sensitivity | High for volatile compounds | Very high for polar and thermolabile compounds |
| Resolution | Excellent | Excellent (especially with UHPLC) |
| Sample Preparation | Requires derivatization for non-volatiles | Minimal, often direct injection |
| Applications | Forensic, environmental, petrochemical | Pharmaceutical, biological, clinical |
| Instrument Cost | Generally lower | Higher initial cost |
In practice, one is chosen for small, volatile compounds, whereas the other is preferred for complex biological or thermally unstable molecules.
5. Advantages of GC
- High Chromatographic Resolution: Exceptional separation efficiency.
- Reproducibility: Reliable retention times and fragmentation patterns.
- Established Spectral Libraries: Thousands of reference spectra make identification fast and accurate.
- Cost-Effectiveness: Lower maintenance and operation costs.
It remains the benchmark for confirmatory analysis in forensic and environmental labs.
6. Advantages of LC
- Broad Analyte Range: Ideal for non-volatile and polar compounds.
- Soft Ionization Techniques: Preserve molecular ions, aiding identification.
- High Sensitivity: Capable of detecting trace-level analytes in complex matrices.
- Compatibility with Biological Samples: Useful in pharmacokinetics and metabolomics.
It is increasingly favored in pharmaceutical, proteomic, and clinical applications due to its flexibility and precision.
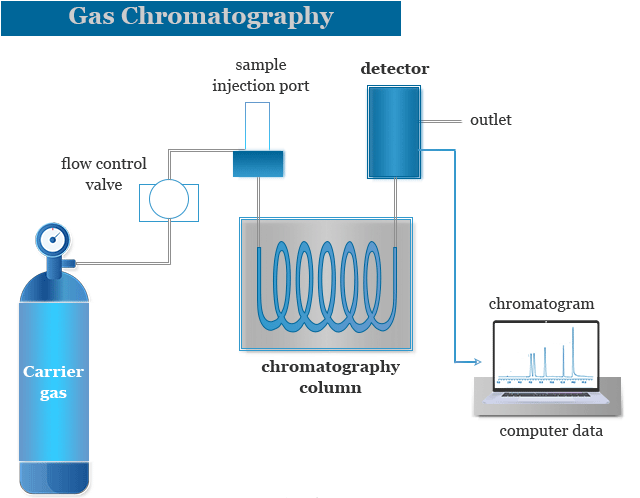
7. Choosing the Right Technique for Your Research
Selecting between the two depends on several key factors:
- Compound volatility and thermal stability:
If the analyte is volatile, GC is preferred. Non-volatile or sensitive compounds are better suited to LC. - Matrix complexity:
LC performs better for biological samples, while GC excels with environmental and organic solvents. - Analytical objective:
For quantitative analysis of trace volatile compounds — GC. For complex molecule identification — LC.
In many research settings, both methods are complementary, providing comprehensive insight when used together.
8. Emerging Trends in Analytical Mass Spectrometry
The evolution of mass spectrometry continues to shape analytical research chemistry. Some of the latest trends include:
- High-Resolution MS (HRMS): Enables ultra-precise mass detection for isomer differentiation.
- Tandem MS (MS/MS): Enhances selectivity and confidence in identification.
- Ambient Ionization Techniques (DESI, DART): Allow direct sample analysis with minimal preparation.
- Automation and AI Integration: Machine learning algorithms now assist in pattern recognition and compound identification.
These advancements are expanding both capabilities, making them even more vital in analytical and forensic science.
9. Applications in Forensic and Environmental Research
Both play critical roles in the identification of unknown compounds and monitoring of environmental pollutants.
- Forensic Analysis: Detection of drugs, metabolites, and controlled substances.
- Environmental Chemistry: Monitoring air, soil, and water contaminants.
- Pharmaceutical R&D: Compound purity, degradation profiling, and bioavailability studies.
- Food Safety: Identifying trace contaminants, flavor compounds, and additives.
The integration of these techniques into routine testing ensures data reliability and supports regulatory compliance.
10. The Future of Chromatography in Analytical Research Chemistry
As instrumentation becomes more advanced, hybrid systems like GC/MS and LC/MS are redefining performance standards. These combinations offer multidimensional separation and improved detection sensitivity.
Furthermore, miniaturization and portable MS instruments are expanding applications into field-based analysis, on-site testing, and rapid screening environments.
The future of analytical research chemistry will rely on automation, sensitivity, and intelligent data interpretation — and both will remain at the core of this evolution.
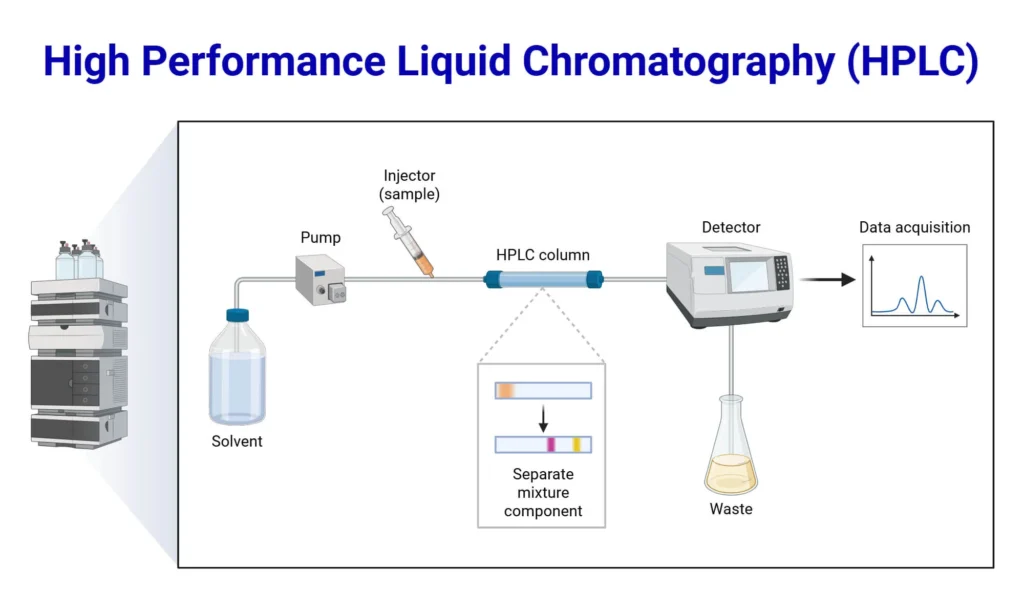
Conclusion
They are both indispensable techniques in analytical and research chemistry, each with its own strengths.
- GC offers unmatched accuracy for volatile, thermally stable compounds.
- LC delivers versatility and sensitivity for complex, polar molecules.
For researchers and laboratories, understanding when and how to apply each method ensures optimal analytical outcomes. Together, they continue to drive innovation across forensic science, pharmaceuticals, environmental testing, and beyond.
Both methods not only define modern analytical and research chemistry, they represent the foundation of precise, reliable scientific discovery.

[…] due to subtle structural differences, which can affect how they bind to receptors, appear in chromatographic analysis, or metabolize in biological […]